Each simple substance according to its physical properties is divided into metals and non-metals. How to distinguish metals from non-metals? Some of them are easily identified visually: hydrogen is a non-metal, and iron is a metal. But, in order to avoid a possible error in the classification, it is better to determine most of the elements by signs.
-
All metals, when kept at normal temperatures, are solids. The exception to this rule is mercury. All metals have a metallic luster and are good thermal and electrical conductors. Almost all metals are malleable when subjected to physical impact.
-
Non-metals are characterized by much greater differences compared to metals. So, they can be liquid (bromine), solid (sulphur), or gaseous (hydrogen). They are also poor thermal and electrical conductors.
-
Metals and nonmetals have different structures. Non-metals are characterized by a large number of free atoms at the outer level compared to metals. The latter is characterized by a non-molecular structure – a crystal lattice.
-
Non-metals have a large redox potential and electronegativity.
-
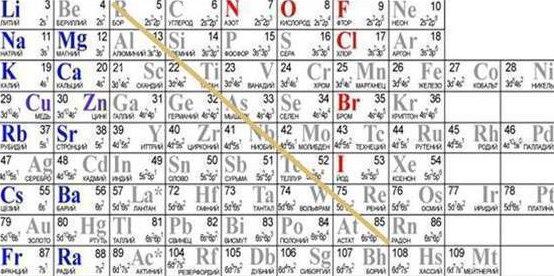
How to distinguish a metal from a non-metal without studying their physical and chemical properties? To do this, you can use the periodic table: you should mentally draw a line from boron to astatine. The left side of the bottom table shows metals. They can also be found in side subgroups located at the top of the ladder. The remaining parts of the main subgroups contain non-metals.
- In addition, many tables are made in color. Non-metals are indicated in red in such tables, and metals are indicated in green and black.
- Do not forget also about the existence of amphoteric elements, which in various chemical reactions exhibit the properties of metals or non-metals. In the periodic table, they are highlighted with hatching. They are called semimetals. Such substances have a metallic luster and are weak electrical conductors.
In addition, there are also metal alloys in industry, which were obtained by fusing metal with non-metals or other metals, for example, cast iron, steel, bronze, brass.
Alloys can be made from two or more components. However, not all components have a good interaction with each other, so it is not always possible to obtain the desired alloy. So, for example, iron and lead, lead and zinc do not alloy with each other, since in the liquid state they do not form a solution.
A prerequisite for obtaining alloys is the formation of a liquid homogeneous solution. The resulting alloys have properties that are different from those of the components from which they were formed.
Pure metals are rarely used in industry, because they do not always have the required properties and economy.
There is another way to distinguish metals from non-metals: a magnet. However, it should be noted that the magnet is a limited tool in the determination of metals, since only non-precious metals have the properties of attraction to it. So, for example, cast iron, steel, iron will be attracted to a magnet, but aluminum, silver, copper will not be attracted. In the same way, you will not be able to check gold at home for authenticity.
Video on how to distinguish metals
How to distinguish slag from metal?
Slags are by-products that result from the following processes:
- Melting of non-ferrous and ferrous metals.
- Combustion of solid fuel.
- Electrothermal sublimation of phosphorus.
Metallurgical slags are melts that cover liquid metal during the metallurgical process. After solidification, the slags are stone-like or glassy substances.
The mineral and chemical composition of slag depends on such factors:
- The composition of the waste ore rock.
- Fuel.
- Type of smelted metal.
- Features of metallurgical processes.
- fuel combustion conditions.
- Slag cooling conditions.
Slag is characterized by its physical properties:
- melting temperature.
- Temperature range of hardening.
- heat capacity.
- Viscosity.
- The ability to dissolve sulfides, oxides, etc.
- certain density.
- certain gas permeability.
The optimum melting temperature of slags is 1100-1200 °C. If the steel is melted at a temperature of 1400-1500 ° C, then the slag should have low viscosity, high mobility and fluidity – these conditions ensure the correct formation of the weld in welding. A very important role is played by how the molten slag solidifies. Slags do not have a strictly defined temperature melting regime. If the temperature rises, the slag becomes less viscous, and if it decreases, then the viscosity increases.
The composition and properties of slags depend on the initial fluxes. The temperature of the submerged metal should be at least 1500-1550 °C, while the temperature of the slags should be 1750 °C.
The question often arises of how to distinguish slag from metal. The main differences are:
- The metal has more liquid and mobility.
- When melted, you can see how the metal boils, which cannot be said about slags.
- Slags are more ductile and have a darker color compared to metal.
- Slags are always lighter than metals.
How to distinguish cast iron from metal?
Cast iron is a metal formed by an alloy of iron and carbon. This material has good casting qualities and is cheap. As a rule, cast iron is used in the engineering industry. In addition, pig iron is the main raw material for steelmaking. Iron ore, fluxes and fuel are used to produce this material.
Often a crack, break or chip can be found in metal structures or parts. In this case, there is a need for repair welding. In order to properly carry out such repairs, you need to know what kind of metals you are dealing with – steel or cast iron. How to distinguish cast iron from metal?
Before proceeding with the study of the surface that needs repair, you should prepare:
- Drill.
- grinding machine.
- Small file or file.
Video on how to distinguish between metals
Order of definition
- First of all, you need to find a place on the surface of the part that is not conspicuous and go through it several times with a small file or needle file. As a result of this, small sawdust is formed on the surface of the metal (or cast iron). They should be taken and rubbed between the fingers. If you are dealing with cast iron, then the fingers will turn into a characteristic black graphite color. For greater clarity, you can take some sawdust and place them between two sheets of white paper and lightly rub the sheets against each other. If you are dealing with steel, then the paper will remain clean.
- You can also determine whether steel is in front of you or cast iron using a grinder. To do this, you should prepare in advance two parts, the composition of which you know for sure, from steel and cast iron. Now you need to turn on the grinder and start sparks from them one by one. Comparing the shape and color of the formed sparks, you need to do the same with the part whose composition is to be determined. The conclusion can be drawn based on the greatest analogy with the samples.
- The sparks that occur during the grinding of steel will fly tangentially along the circumference of the grinding wheel. If the metal contains carbon, that is, you are dealing with cast iron, then the particles that are heated during the grinding process, flying into the air and coming into contact with it, will be oxidized – thus, carbon turns into carbon dioxide. It should also be noted that when a grinding wheel comes into contact with a cast-iron surface, a lot of rather short sparks are always formed. In addition, the sparks generated when grinding cast iron have a bright straw color.
- To determine in the following way, you need to take a drill and insert a drill with a small diameter into it. You should find an inconspicuous place on the part, turn on the drill and drill a little. Since there are significant differences in drilling cast iron parts from drilling steel parts, it is recommended to take two parts made of steel and cast iron and try to drill them. In the process of drilling cast iron, chips are practically not formed. And if they are formed, they will be very small in size, and when rubbed between the fingers, cast iron shavings easily turn into dust. Steel shavings are distinguished by a curled shape and resemble wire. In addition, steel shavings are quite difficult to break with your fingers.
- A lathe can be used in the same way – cast iron chips will look like coarse dust.