Thermodynamics is a science that describes the laws of the transfer of thermal energy from one body to another. With such a transition, it is possible to convert part of the thermal energy into useful work.
Therefore, thermodynamics is the basis for the calculations of any heat engines: from steam engines to some fantastic photonic rockets. Yes, yes, do not be surprised, even when flying into space, thermodynamics is indispensable!
Thermodynamics originated in the 18th century with the first working steam engines. In less than a hundred years, three basic postulates that underlie this science, which are called the laws (or principles) of thermodynamics, have been developed. The laws of thermodynamics are a kind of axioms that are a generalization of vast engineering experience. Therefore, they are accepted without proof. The question "why?" for axioms, as is known, it is not customary to specify. That’s why!
Thermodynamics considers thermodynamic systems, that is, systems that have, among other things, a certain amount of internal energy, which in everyday life we call heat. The first law of thermodynamics is the law of conservation of energy for such systems. According to this law, a thermodynamic system can perform useful work only due to its internal energy, or due to the influx of energy from outside.
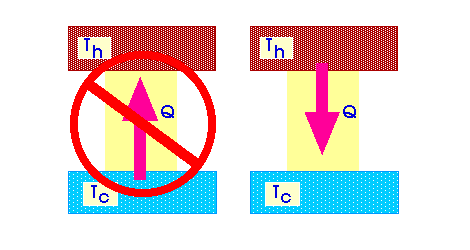
The second law of thermodynamics determines the direction of transition of internal energy between thermodynamic systems. According to this law, internal energy (i.e. heat) can spontaneously transfer in only one direction – from a body with more internal energy to a body with less of it. That is, heat itself can only pass from a hotter body to a colder body. With such a transition, part of the thermal energy can be converted into useful work with the help of specially designed heat engines. The percentage of internal energy converted into useful work is called the coefficient of efficiency.
The second law of thermodynamics does not contradict the existence of refrigerators and air conditioners. Indeed, in order to cool an object, that is, to transfer its heat to a hotter object, energy must be expended. Roughly speaking, any cooler will not work by itself. To do this, you need to connect it to the electrical network.
Both the first and second laws of thermodynamics seem obvious. How to invent and calculate efficient heat engines (or refrigerators) with the help of these obvious laws cannot be told on "one leg". Therefore, take a textbook and "gnaw the granite of science"!